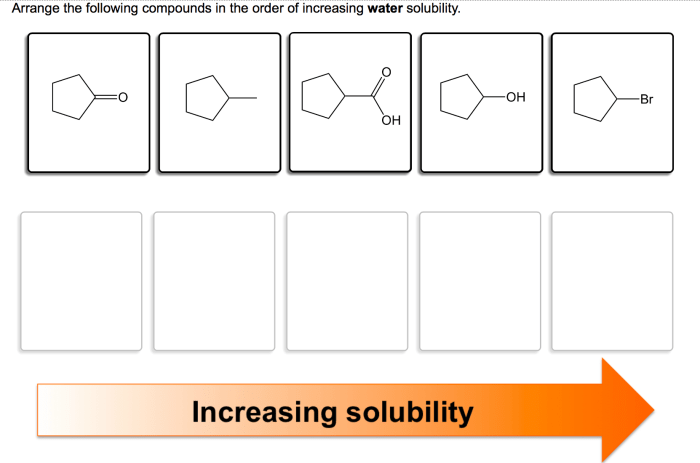
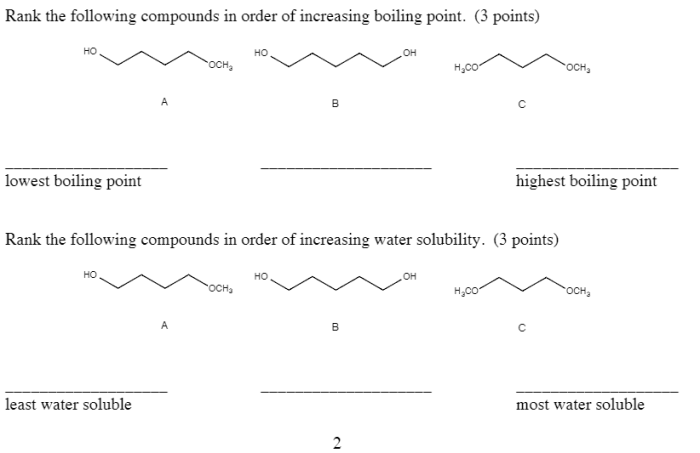
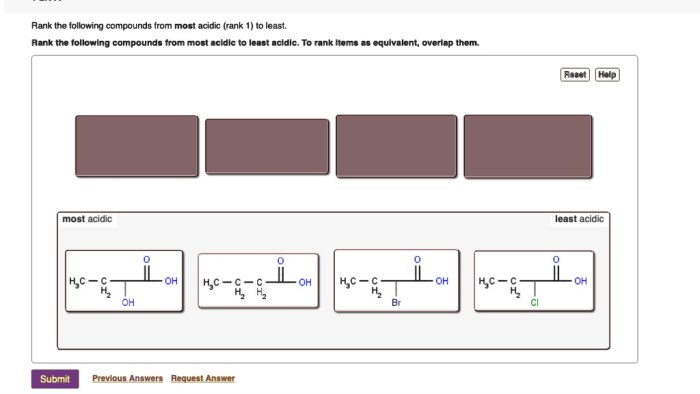
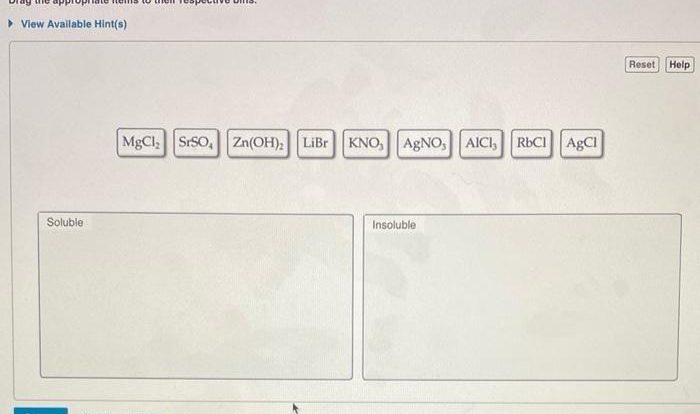
Rank these substances in order of increasing solubility in water. This guide delves into the intricacies of solubility, exploring the factors that govern how substances dissolve in water. From understanding the nature of intermolecular forces to examining practical applications, this comprehensive overview provides a thorough examination of this fundamental chemical property.
Solubility plays a pivotal role in numerous scientific disciplines, influencing drug delivery, water purification, and chemical reactions. By unraveling the mechanisms behind solubility, we gain valuable insights into the behavior of substances and their interactions with water.
Ranking Substances by Solubility: Rank These Substances In Order Of Increasing Solubility In Water.

Solubility is a measure of the ability of a substance to dissolve in a solvent. It is an important concept in chemistry, as it affects the properties and behavior of substances in solution. The solubility of a substance is influenced by several factors, including temperature, pressure, and polarity.Polarity
refers to the distribution of electrical charge within a molecule. Polar molecules have a positive end and a negative end, while nonpolar molecules have a uniform distribution of charge. Intermolecular forces are the forces that act between molecules. Hydrogen bonding, dipole-dipole interactions, and van der Waals forces are the three main types of intermolecular forces.The
solubility of a substance in water is directly related to the strength of the intermolecular forces between the substance and water molecules. Substances that have strong intermolecular forces with water will be more soluble in water than substances that have weak intermolecular forces with water.The
following table ranks the given substances in order of increasing solubility in water:| Substance | Chemical Formula | Polarity | Solubility in Water ||—|—|—|—|| Hexane | C 6H 14| Nonpolar | Insoluble || Benzene | C 6H 6| Nonpolar | Slightly soluble || Ethanol | C 2H 5OH | Polar | Soluble || Sodium chloride | NaCl | Ionic | Highly soluble |Hexane is a nonpolar hydrocarbon that has weak intermolecular forces with water.
As a result, it is insoluble in water. Benzene is also a nonpolar hydrocarbon, but it has a slightly higher solubility in water than hexane because it has a slightly stronger dipole-dipole interaction with water. Ethanol is a polar molecule that has hydrogen bonding with water.
As a result, it is soluble in water. Sodium chloride is an ionic compound that dissolves in water to form ions. As a result, it is highly soluble in water.The solubility of a substance in water is an important property that affects its behavior in solution.
By understanding the factors that affect solubility, we can better understand the properties and behavior of substances in solution.
General Inquiries
What is the significance of solubility in chemistry?
Solubility is a fundamental property that influences chemical reactions, drug delivery, and water purification. Understanding solubility allows chemists to predict the behavior of substances in aqueous environments.
How do intermolecular forces affect solubility?
Intermolecular forces, such as hydrogen bonding, dipole-dipole interactions, and van der Waals forces, determine the strength of the attraction between solute molecules and water molecules. Stronger intermolecular forces lead to higher solubility.