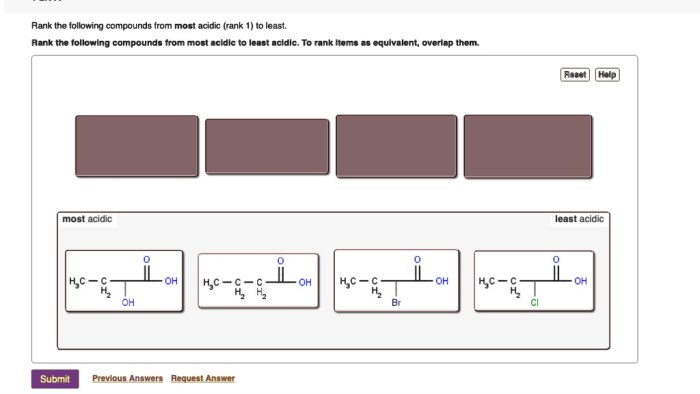
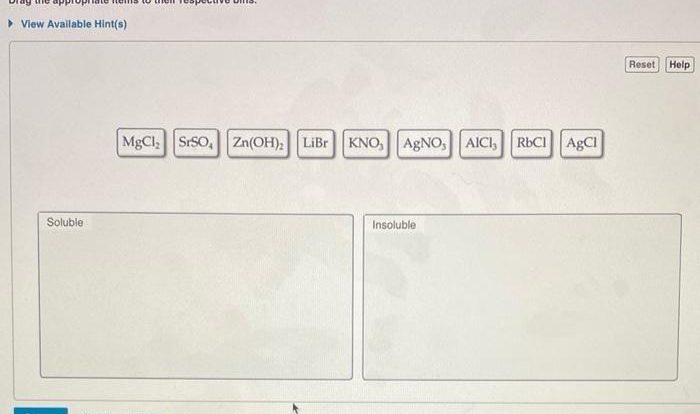
Valence electrons and ions worksheet answer key offers an in-depth examination of the fundamental concepts surrounding valence electrons and ions, providing a comprehensive guide for students and educators alike.
This meticulously crafted resource delves into the nature of valence electrons, their significance in chemical reactions, and their relationship to the periodic table. Furthermore, it explores the formation of ions, their classification, and the crucial role valence electrons play in this process.
Valence Electrons and Ions

In chemistry, valence electrons are the electrons in the outermost shell of an atom. These electrons are involved in chemical reactions and determine the chemical properties of an element.
Valence Electrons, Valence electrons and ions worksheet answer key
- Valence electrons are the electrons in the outermost energy level of an atom.
- The number of valence electrons determines the chemical reactivity of an element.
- Elements with the same number of valence electrons tend to have similar chemical properties.
For example, the element sodium has one valence electron, and it is a highly reactive metal. The element chlorine has seven valence electrons, and it is a highly reactive nonmetal.
The periodic table is arranged in such a way that elements with the same number of valence electrons are in the same column.
Ions
Ions are atoms that have lost or gained electrons. When an atom loses an electron, it becomes a positively charged ion, called a cation. When an atom gains an electron, it becomes a negatively charged ion, called an anion.
The formation of ions is driven by the desire of atoms to achieve a stable electron configuration. Atoms with a full valence shell are the most stable.
The relationship between valence electrons and ion formation is that the number of valence electrons determines the charge of the ion.
- Atoms with one valence electron tend to lose that electron and form cations with a charge of +1.
- Atoms with seven valence electrons tend to gain one electron and form anions with a charge of -1.
- Atoms with two valence electrons tend to lose both electrons and form cations with a charge of +2.
- Atoms with six valence electrons tend to gain two electrons and form anions with a charge of -2.
Popular Questions: Valence Electrons And Ions Worksheet Answer Key
What are valence electrons?
Valence electrons are the electrons in the outermost energy level of an atom, which determine its chemical properties and reactivity.
How are ions formed?
Ions are formed when atoms gain or lose electrons, resulting in a change in their overall electrical charge.
What is the relationship between valence electrons and ion formation?
The number of valence electrons an atom has determines its tendency to form ions and the type of ion it forms (cation or anion).