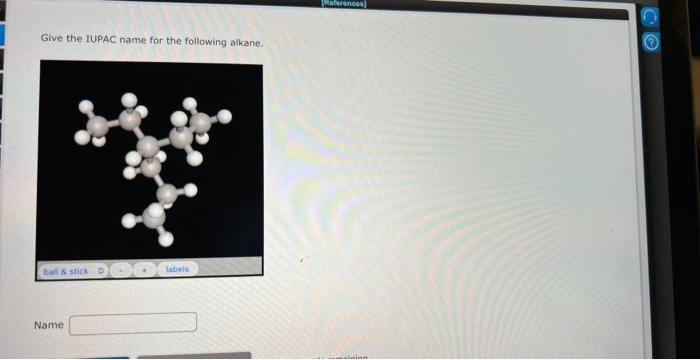
Give the iupac name of the following alkane. – In the realm of organic chemistry, the International Union of Pure and Applied Chemistry (IUPAC) nomenclature system reigns supreme, providing a standardized and systematic approach to naming alkanes. This comprehensive guide delves into the intricacies of IUPAC nomenclature, empowering you to decipher and assign precise names to these saturated hydrocarbons.
As we embark on this journey, we will unravel the defining characteristics of alkanes, explore their molecular structures, and master the step-by-step procedure for naming them according to IUPAC rules. Along the way, we will uncover the nuances of common and systematic names, equipping you with the knowledge to navigate the world of alkane nomenclature with confidence.
IUPAC Nomenclature of Alkanes: Give The Iupac Name Of The Following Alkane.
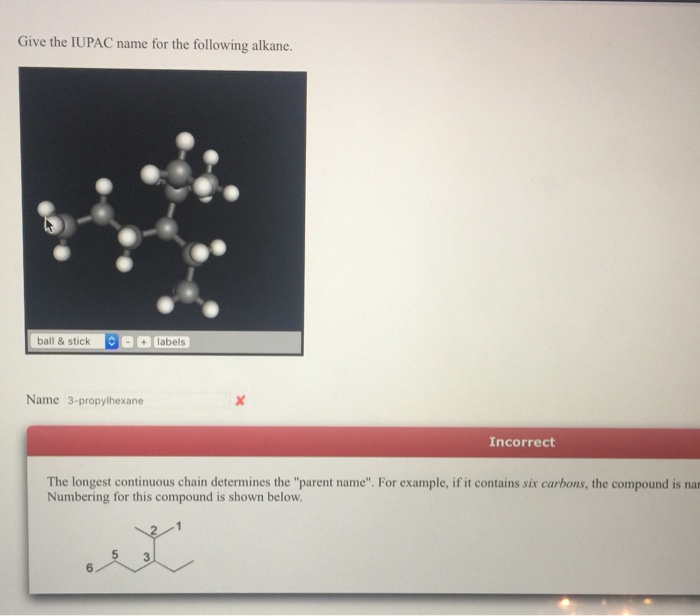
The International Union of Pure and Applied Chemistry (IUPAC) system for naming alkanes is a set of rules that assigns a unique name to each alkane. The name is based on the structure of the alkane, and it reflects the number and arrangement of carbon atoms in the molecule.
Identifying Alkanes
Alkanes are hydrocarbons that contain only carbon and hydrogen atoms. They are saturated hydrocarbons, meaning that all of the carbon atoms are bonded to four other atoms. Alkanes are typically colorless, odorless, and tasteless.
Alkanes can be identified by their structural features. Alkanes have a general formula of CnH2n+2, where n is the number of carbon atoms in the molecule. The carbon atoms in alkanes are arranged in a chain, and the hydrogen atoms are attached to the carbon atoms in a tetrahedral arrangement.
Alkane Structure
The molecular structure of alkanes is relatively simple. The carbon atoms in alkanes are arranged in a chain, and the hydrogen atoms are attached to the carbon atoms in a tetrahedral arrangement. The tetrahedral arrangement of the hydrogen atoms around the carbon atoms gives alkanes their characteristic three-dimensional shape.
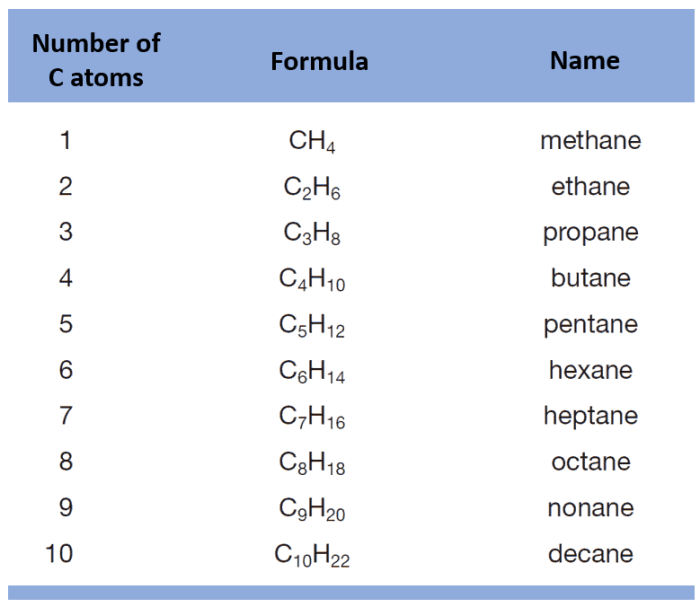
The number of carbon atoms in an alkane determines the type of alkane. Alkanes with one carbon atom are called methanes, alkanes with two carbon atoms are called ethanes, alkanes with three carbon atoms are called propanes, and so on.
IUPAC Naming Procedure, Give the iupac name of the following alkane.
The IUPAC system for naming alkanes is based on the following rules:
- The root of the name is based on the number of carbon atoms in the molecule.
- The suffix
ane is added to the root to indicate that the molecule is an alkane.
- The prefixes di-, tri-, tetra-, etc., are used to indicate the number of branches on the alkane chain.
- The branches are named in alphabetical order.
- The numbers of the carbon atoms to which the branches are attached are included in the name.
Common and Systematic Names
Alkanes can have both common names and systematic IUPAC names. Common names are often used for simple alkanes, such as methane, ethane, and propane. Systematic IUPAC names are used for more complex alkanes.
There are several advantages to using systematic IUPAC names for alkanes. Systematic IUPAC names are unique, and they provide a clear and concise way to identify alkanes. Systematic IUPAC names are also used in scientific literature, so they are important for scientists to know.
Essential Questionnaire
What is the IUPAC system for naming alkanes?
The IUPAC system is a set of rules established by the International Union of Pure and Applied Chemistry for naming organic compounds, including alkanes. It involves identifying the parent chain, assigning prefixes based on the number of carbon atoms, and using suffixes to indicate the presence of functional groups.
How do I identify the parent chain in an alkane?
The parent chain is the longest continuous chain of carbon atoms in the molecule. It forms the basis for the root name of the alkane.
What are the prefixes used in IUPAC alkane nomenclature?
The prefixes used in IUPAC alkane nomenclature are: meth- (1 carbon), eth- (2 carbons), prop- (3 carbons), but- (4 carbons), pent- (5 carbons), hex- (6 carbons), hept- (7 carbons), oct- (8 carbons), non- (9 carbons), and dec- (10 carbons).